Top Fintech trends 2025: The roadmap to smarter financial solutions
Embrace the top fintech trends 2025: from embedded finance to Amazonization, learn how financial service companies make smart decisions for a better future.

Exploring the innovations shaping the future of biotechnology and pharmaceutical research in 2024.
The biopharma industry is experiencing a rapid evolution as new technologies and methodologies emerge. In 2024, the global biotech market will reach $465.9 billion, driven by groundbreaking advancements and innovative approaches.
Without further ado, this midyear update explores these “advancements and innovative approaches” shaping the sector in 2024. And, most likely, continue to shape the industry way beyond. Here, we aim to provide a comprehensive overview of the latest developments as of July 2024, offering insights into how they shape biopharma’s future.
Unsurprisingly, Artificial intelligence (AI) is revolutionizing the biotech industry. It enhances drug discovery, optimizing clinical trials, and advancing precision medicine. As AI technology matures, its integration into biotech processes yields tangible results, marking a significant leap forward.
According to the most recent statistics, the integration of AI within the drug discovery market is moving significantly. The more companies working in drug discovery use AI, the greater demand for the technology emerges. As a result, the market size explodes (see Fig.1). Figure 1. AI in drug discovery market size, 2023 to 2032 in USD million
Figure 1. AI in drug discovery market size, 2023 to 2032 in USD million
AI changes drug discovery by analyzing vast datasets to identify potential drug candidates and optimize development timelines. The integration of AI in this field represents a significant leap forward, providing unprecedented speed and accuracy in identifying new therapeutic compounds. Here are some indicative aspects to mention:
AI’s role in drug discovery is shifting from mere adoption to achieving tangible successes in drug development, marking a new phase in the industry’s evolution. As the technology matures, the focus is increasingly on delivering real-world results.
Oncology professionals are among the early adopters of AI. Experts in the field use the technology to identify patterns in patient data and optimize treatment strategies. That is exactly why AI in the oncology market is currently on the rise (see Fig.2).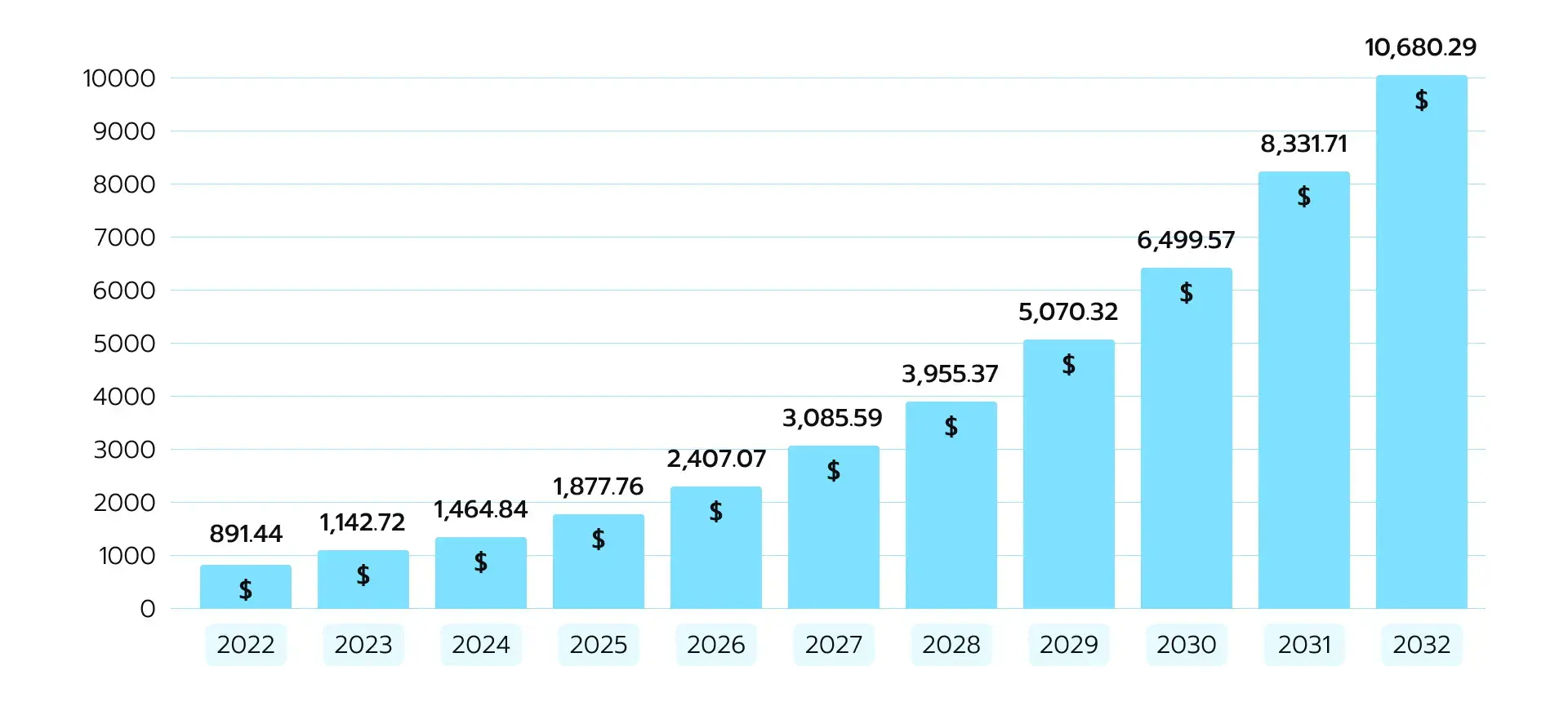 Figure 2. AI in oncology market size, 2023 to 2032 in USD million
Figure 2. AI in oncology market size, 2023 to 2032 in USD million
These are some of the key aspects making AI so beneficial to oncology:
AI is a game-changer in oncology. It offers more precise and personalized treatment strategies that significantly improve patient care and enhances early diagnosis, helping to recognize cancer at stages that might otherwise be missed by traditional methods.
Real World Evidence (RWE) is becoming key for biotech firms. It is redefining drug development. And it does so by using existing data from electronic health records (EHR) and real-world practices. In such a case, there is definitely a bright future for RWE in biopharma (see Fig.3). Figure 3. Global RWE solutions market in USD billion
Figure 3. Global RWE solutions market in USD billion
When it comes to particular things RWE offers to biopharma, let’s take a look at these principal indications:
RWE offers biotech companies a competitive edge by enhancing the efficiency and effectiveness of drug development. It ultimately leads to more personalized and patient-centric treatments.
Integrating genomic, transcriptomic, proteomic, and metabolomic data provides a comprehensive understanding of biological systems, essential for advancing precision medicine. What is more, working with multi-omic data represents a pretty lucrative opportunity, with a market rising at about 15% per annum (see Fig. 4). Figure 4. Multi-omics market statistics
Figure 4. Multi-omics market statistics
This multi-omic approach combines multiple types of omic data to create a holistic view of molecular mechanisms within biological systems.
Integrating multi-omic data with AI boosts biological research potential. AI algorithms can analyze extensive and complex datasets, revealing patterns and insights that traditional methods might overlook.
Enhanced by AI, multi-omic data is crucial for comprehensive biological research and precision medicine. This powerful combination offers profound insights into disease mechanisms and potential treatments.
Overall, though it might sound like a cliche, the evidence truly shows how AI is reshaping the biopharma industry. The technology drives significant advancements in drug discovery, clinical trials, and personalized medicine.
The integration of AI, advancements in RWE, and multi-omic data have changed the industry as never before. Now, people working in the given segment have on their hands unprecedented opportunities for research and patient care.
Bioinformatics is undergoing rapid advancements. Coupled with innovative technologies, the industry presents deeper insights into biological systems on almost a daily basis. These trends have a direct impact on other important sectors, including healthcare, agriculture, and environmental studies. Specifically, understanding cellular and microbial heterogeneity is a way to make things a lot better for the aforementioned industries. Let’s see how it’s happening right now.
Single-cell sequencing is all about understanding cellular heterogeneity by providing unprecedented resolution at the individual cell level. In layman’s words, single-cell sequencing helps understand the differences between individual cells by giving very detailed information about each cell. This advanced technique is crucial for identifying and studying rare cell types, which is particularly important in the context of tumor heterogeneity and disease progression.
Single-cell sequencing is a powerful tool for advancing cancer research and personalized medicine.
Metagenomics digs into the genetic material of microbial communities in diverse environments. Essentially, it studies the DNA of all the microbes living in different environments, like soil, water, or the human body. At the moment, the metagenomic market experiences its best years (see Fig. 5). Figure 5. U.S. metagenomic sequencing market size 2023 to 2032 in USD million
Figure 5. U.S. metagenomic sequencing market size 2023 to 2032 in USD million
Metagenomics has significant implications for understanding the human microbiome and the biodiversity of ecosystems. Here are some notable moments to mention:
Metagenomics offers a deeper understanding of microbial ecosystems. Its ability to analyze complex microbial communities is essential for developing new therapies, improving agricultural practices, and enhancing environmental conservation efforts.
Putting all things together, bioinformatics is definitely a rapidly evolving field with transformative potential across various sectors. Technologies like single-cell sequencing and metagenomics are at the forefront. They drive significant progress in cancer research, personalized medicine, healthcare, agriculture, and environmental studies. Evidently, embracing these trends is crucial for pushing the boundaries of scientific discovery and application.
In a nutshell, personalized medicine is based on developing treatments for individual genetic profiles. To give you an idea of what it means, here is a hypothetical scenario. Imagine two people with the same type of cancer. Personalized medicine looks at each person’s unique genetic makeup. One person might have a gene that makes their cancer grow faster, so their doctor gives them a treatment that specifically targets that gene. The other person might have a different gene that affects how they respond to a drug, so they get a different treatment. This way, each person gets a treatment tailored to their own genetics.
In such a context, personalized medicine enhances the effectiveness of therapies and reduces adverse effects. It offers a more precise and patient-centric treatment paradigm.
As with the example above, a crucial part of personalized medicine per se is precision oncology. At the moment, there are about 20 million new cancer cases globally every year. In other words, precision oncology can be a game-changer in bringing the number of deaths from cancer down. To grasp the scope of the industry, here is a segmentation (see Fig.6). Figure 6. Precision oncology market share by region
Figure 6. Precision oncology market share by region
Precision oncology makes sure that individual cancer treatments are more targeted and effective. In most cases, experts can achieve that by following these aspects:
Personalized medicine transforms cancer treatment by offering more targeted and effective therapies. By studying a person’s genes and how they respond to drugs, doctors can tailor treatments specifically for them. This results in better outcomes and fewer side effects.
Functional genomics helps scientists understand what each gene does and how it’s controlled. For example, this knowledge is crucial for CRISPR-based gene editing, which can precisely change or fix genes to treat diseases. The evidence shows functional genomics market will more than triple in the next decade (see Fig.7). Figure 7. Global functional genomics market
Figure 7. Global functional genomics market
Functional genomics is essential for deciphering the complex mechanisms underlying gene function and regulation. Take a look at some of its crucial components:
Functional genomics is crucial for advancing gene therapies and understanding complex genetic mechanisms. The insights gained from this field are essential for developing precise gene-editing technologies and uncovering the regulatory functions of non-coding RNAs, driving forward the potential for innovative treatments.
Advancements in personalized medicine are setting new standards in healthcare, particularly in cancer treatment and gene therapy. These advancements show how personalized medicine can improve patient care by offering treatments designed specifically for each person.
Integrating genomic, transcriptomic, proteomic, and metabolomic data provides a comprehensive understanding of biological systems. Here’s how it all works. To understand how a disease works, scientists look at different types of data, like genes, proteins, and metabolism. For example, by studying all these areas together, they can get a complete picture of how a disease affects the body and create more precise treatments. This helps doctors choose the best treatment for each patient.
Multi-omic data integration is essential for comprehensive biological research and precision medicine. It offers valuable insights into the intricate interactions within biological systems. It aids in the discovery of new biomarkers and therapeutic targets.
Network biology views biological systems as interconnected networks, identifying key signaling pathways and drug-target interactions. At the moment, the network biology market is expanding really fast (see Fig.8). Figure 8. Network biology market segmentation by region
Figure 8. Network biology market segmentation by region
Network biology is vital for understanding how various components of a biological system interact and influence each other.
Network biology offers a systemic approach to understanding and treating diseases. Viewing biological systems as interconnected networks provides insights into signaling pathways and drug targets, facilitating the development of more effective therapeutic strategies.
Integrating multi-omic data and the systemic approach of network biology are transforming the landscape of biological research and medicine. Multi-omic data integration offers a holistic view of molecular mechanisms, enhancing the identification of biomarkers and therapeutic targets.
Network biology, by understanding biological systems as interconnected networks, provides crucial insights into signaling pathways and drug targets. Together, these advancements are paving the way for precision medicine and innovative treatments, offering new hope for better patient outcomes.
 The field of genomic research is rapidly evolving with new technologies that enhance genetic data analysis’s accuracy, security, and transparency. These innovations are crucial for advancing our understanding of complex genetic structures and ensuring the integrity of genomic data sharing.
The field of genomic research is rapidly evolving with new technologies that enhance genetic data analysis’s accuracy, security, and transparency. These innovations are crucial for advancing our understanding of complex genetic structures and ensuring the integrity of genomic data sharing.
Long-read sequencing technology changes genomic research by giving us more accurate and complete pictures of genomes. This helps scientists understand DNA better and find important details they might miss with other methods.
Long-read sequencing enhances genomic research by providing detailed and accurate genetic information. Its ability to produce high-quality genome assemblies and detect structural variants makes it indispensable for advancing our understanding of genetics and disease.
Blockchain is not only about making secure financial transactions. Even though it is mainly associated with cryptocurrencies, in reality, the technology has a far greater potential. In such a case, in genomics, blockchain technology is the approach that makes some great promises (see Fig.9). Figure 9. Blockchain in the Genomics Market
Figure 9. Blockchain in the Genomics Market
Blockchain technology enhances security and transparency in genomic data sharing, addressing critical data privacy and integrity issues. The technology ensures that genomic data is securely shared and reliably managed, fostering greater collaboration and trust in the research community.
The adoption of new technologies in genomic research, such as long-read sequencing and blockchain, is transforming the field by enhancing accuracy, security, and transparency. These advancements are paving the way for more precise and reliable genomic research.
While we laid the groundwork for key biotech trends, there are some of them that were intentionally left behind. Namely, technologies like AI, bioinformatics, and blockchain prove to reshape biopharma at its core. Yet, the trends we present further are the ones that also have an impact. However, it is not as great as the one of AI, for instance.
So, let’s take a look at some emerging trends in biotech.
Drug repurposing finds new ways to use existing medicines to treat different diseases. For example, a drug originally for high blood pressure might be found to help with Alzheimer’s disease. This method is faster and cheaper because we already know the drug is safe.
Also, here are some notable advantages drug repurposing brings:
Drug repurposing is a strategic approach to accelerate drug discovery and reduce costs. This method offers a faster and more economical pathway to treatment development by re-evaluating existing drugs for new therapeutic purposes.
Digital pathology uses computer technology to combine images of tissue samples with molecular data. At the moment, the digital pathology market is attracting some significant investment (see Fig.10). Figure 10. Digital pathology market size
Figure 10. Digital pathology market size
Digital pathology helps doctors analyze and diagnose diseases, like cancer, more accurately and quickly. In most cases, these are the approaches used:
Digital pathology revolutionizes disease diagnosis and research with advanced imaging and data integration. By automating the analysis of pathology images and combining them with molecular data, digital pathology offers more accurate, efficient, and comprehensive diagnostic solutions.
Bioprinting and tissue engineering are emerging fields that create complex tissue structures using 3D printing technologies. These advancements have significant implications for regenerative medicine and organ transplantation. Here’s how the bioprinting market is performing at the moment (see Fig.11). Figure 11. 3D bioprinting market size in USD billion
Figure 11. 3D bioprinting market size in USD billion
Essentially, bioprinting is all about these two aspects:
Bioprinting and tissue engineering are pushing the boundaries of regenerative medicine, offering innovative solutions for tissue repair and organ replacement.
Personalized vaccines, developed using individual genetic information, significantly advance immunotherapy. There is great demand within the personalized vaccines market (see Fig.12).
 Figure 12. Global personalized cancer vaccines market
Figure 12. Global personalized cancer vaccines market
Personalized vaccines have the most promise in these areas:
Personalized vaccines represent a new frontier in immunotherapy, offering tailored treatments that improve efficacy and patient outcomes.
Synthetic biology integrates biology and engineering to design and create new biological components, devices, and systems. For example, scientists can design bacteria that produce medicine or clean up pollution.
At the moment, synthetic biology is widely used to access these particular industries (see Fig.13). Figure 13. Synthetic biology market by application
Figure 13. Synthetic biology market by application
Synthetic biology can change various industries, including healthcare, agriculture, and energy. Here’s how:
The biotechnology sector is advancing rapidly with emerging trends such as drug repurposing, digital pathology, bioprinting, personalized vaccines, and synthetic biology. These innovations are driving significant progress in drug discovery, disease diagnosis, and personalized medicine, offering new hope for improved healthcare solutions.
There is a massive difference between the hypothetical and actual benefits that the aforementioned trends can bring. Essentially, one thing is to indicate what particular technology can bring in theory. And a completely different thing to see how it pans out in a real-life setting. Here are some actual examples of how trends we examined were actually applied.
Thalidomide, initially developed as a sedative and treatment for morning sickness in pregnant women, was later repurposed to treat multiple myeloma and leprosy. The drug’s repurposing significantly reduced development costs and accelerated its availability for new therapeutic uses.
PathAI has developed machine learning algorithms to analyze pathology slides to detect cancerous cells. This AI-powered approach significantly enhances diagnostic accuracy and speed.
CRISPR technology successfully treated sickle cell anemia by correcting the defective gene in hematopoietic stem cells. This groundbreaking therapy has shown promise in clinical trials.
Researchers at Wake Forest Institute for Regenerative Medicine bioprinted human skin grafts for burn victims, demonstrating the potential of bioprinting for complex tissue fabrication.
BioNTech’s personalized cancer vaccine for melanoma patients has shown significant promise in clinical trials, enhancing the immune response against tumor-specific antigens.
Ginkgo Bioworks has engineered custom organisms for various applications, including bacteria that produce biofuels and enzymes for industrial processes, showcasing the vast potential of synthetic biology.
Flatiron Health uses RWE from electronic health records to support oncology drug development, helping to shorten development timelines and improve patient outcomes.
These real-case examples demonstrate the transformative power of emerging biotechnologies. From repurposing existing drugs and advancing diagnostic accuracy to engineering new biological systems and enhancing personalized medicine, these innovations pave the way for a new era in healthcare and research. Embracing these technologies will continue to drive progress and improve outcomes across the biotechnology sector.
Biopharma is at the forefront of technological innovation, leveraging AI, bioinformatics, and personalized medicine to drive progress. As we move forward in 2024, integrating these technologies continue reshaping biopharma, offering new possibilities for treatment and disease management.
The biotechnology sector is on the cusp of a transformative era, driven by groundbreaking technologies such as drug repurposing, digital pathology, gene editing, bioprinting, personalized vaccines, synthetic biology, and real-world evidence trials.
There is enough evidence and practical cases showing how theory turns into practice. Avenga is the company actively working with some of the technologies above. Contact us to learn more about our services linked to biopharma.
Ready to innovate your business?
We are! Let’s kick-off our journey to success!