Beyond the stethoscope: Top healthcare technology trends 2025
Embrace the most prominent healthcare technology trends shaping the medical sector in 2025 and beyond. Learn about the top technology and solutions that are changing how we view health.

Beating the clock: how AI can expedite recruitment timelines in clinical trials.
Over the years, patient recruitment has been one of the toughest challenges for research teams, with low enrollment rates contributing to costly delays and hampering the pace of medical innovation. Estimates suggest that over 80% of clinical trials fail to meet their initial enrollment timelines. This persistent challenge begs the question: How can Artificial Intelligence (AI), with its vast and continually expanding capabilities, help recruit a sufficient number of participants in a timely manner? In this article, we will take a closer look at its numerous high-impact applications that drive more efficient patient recruitment and retention strategies.
Successful patient recruitment is vital to the validity of clinical trials. Without a sufficient number of qualified participants, studies risk costly delays or premature termination after years of upfront investment. More than decade ago, 11% of clinical trial sites failed to recruit a single patient, while 37% enrolled an insufficient quantity of participants, a research shows. Nevertheless, the situation appears to be gradually improving.
Although enrollment issues are a leading factor in premature termination of clinical trials, there has been a drop in the number of trials ending due to recruitment problems, Clinical Trials Arena suggests. In fact, in a period from 2010 to 2021, there was a steady decline in the number of enrollment-related terminations of clinical trials (see Fig. 1).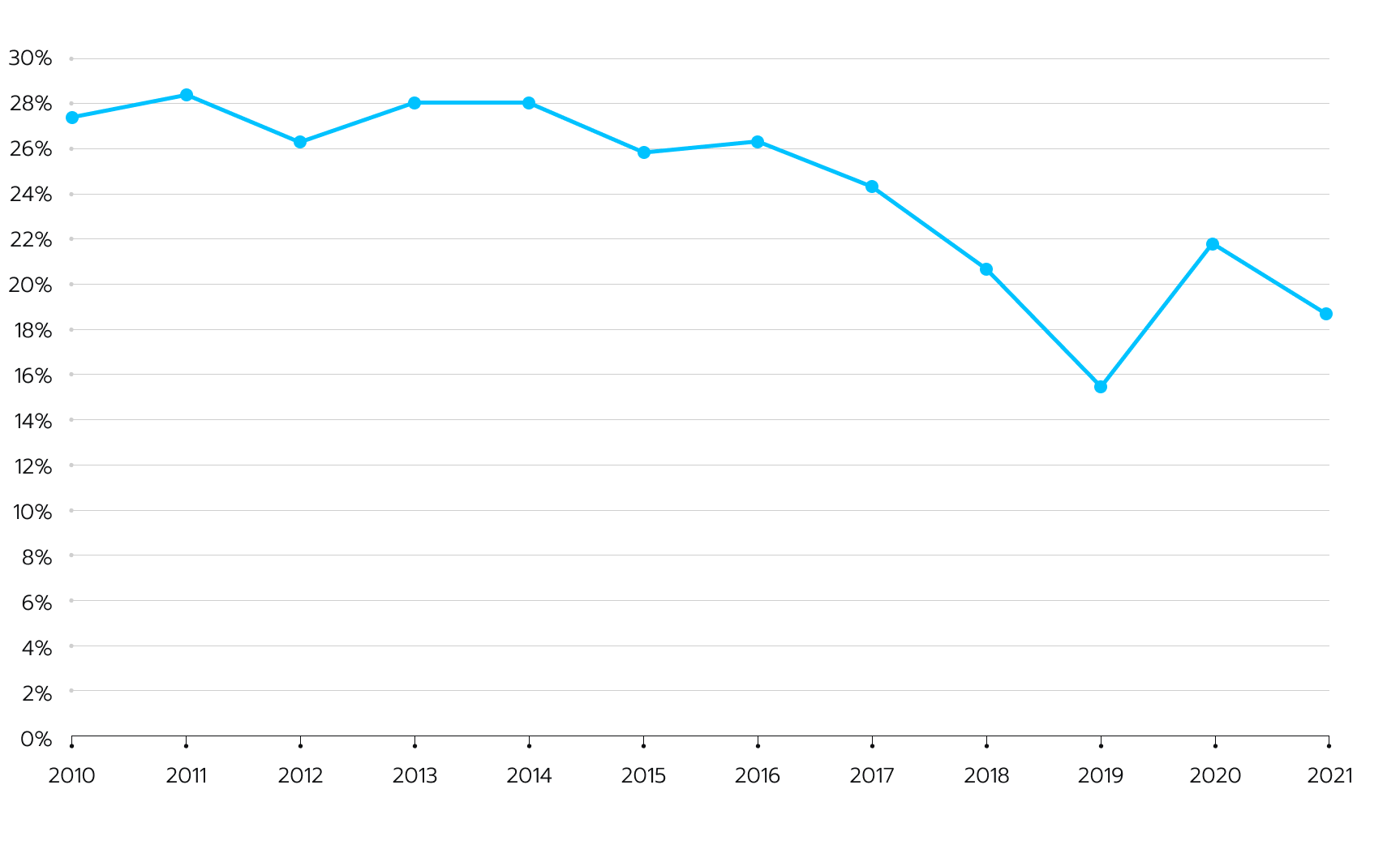 Figure 1. The number of clinical trials terminated partly due to low accrue rates has decreased throughout a decade, according to GlobalData insights.
Figure 1. The number of clinical trials terminated partly due to low accrue rates has decreased throughout a decade, according to GlobalData insights.
The pharmaceutical industry, regulatory bodies, and academic institutions have made concerted efforts to improve patient recruitment strategies and leverage technological advancements. The widespread adoption of decentralized clinical trials (DCTs) has been a game-changer in this regard.
The use of decentralized clinical trials (DCTs) has reduced the burden on participants and increased accessibility to clinical studies through remote monitoring, telemedicine, and mobile health technologies. These technologies have facilitated recruitment efforts and propelled retention rates, as participants are more likely to remain engaged throughout the study duration when the trial is more convenient and accessible.
Moreover, the integration of digital platforms and social media has altered the way clinical trial recruitment is conducted. Sponsors and investigators can now leverage data analytics and targeted advertising to more effectively identify and reach potential participants. These targeted recruitment strategies have proven more efficient and cost-effective than traditional offline methods, such as physician referrals, a study underlines.
Discover how we built an end-to-end patient experience tool for Klara, one of the leading digital health companies in the U.S. Success story
In spite of the favorable trajectory driven by novel recruitment approaches and technologies, numerous essential challenges remain when it comes to patient recruitment. Here is their brief overview:
The concept that describes the gradual attrition of participants from initial screening to trial completion is termed the recruitment funnel. It quantifies the dropout rates across various stages of the recruitment process, beginning with the site’s projected pool of potential participants and culminating in those who successfully complete the clinical trial (see Fig. 2).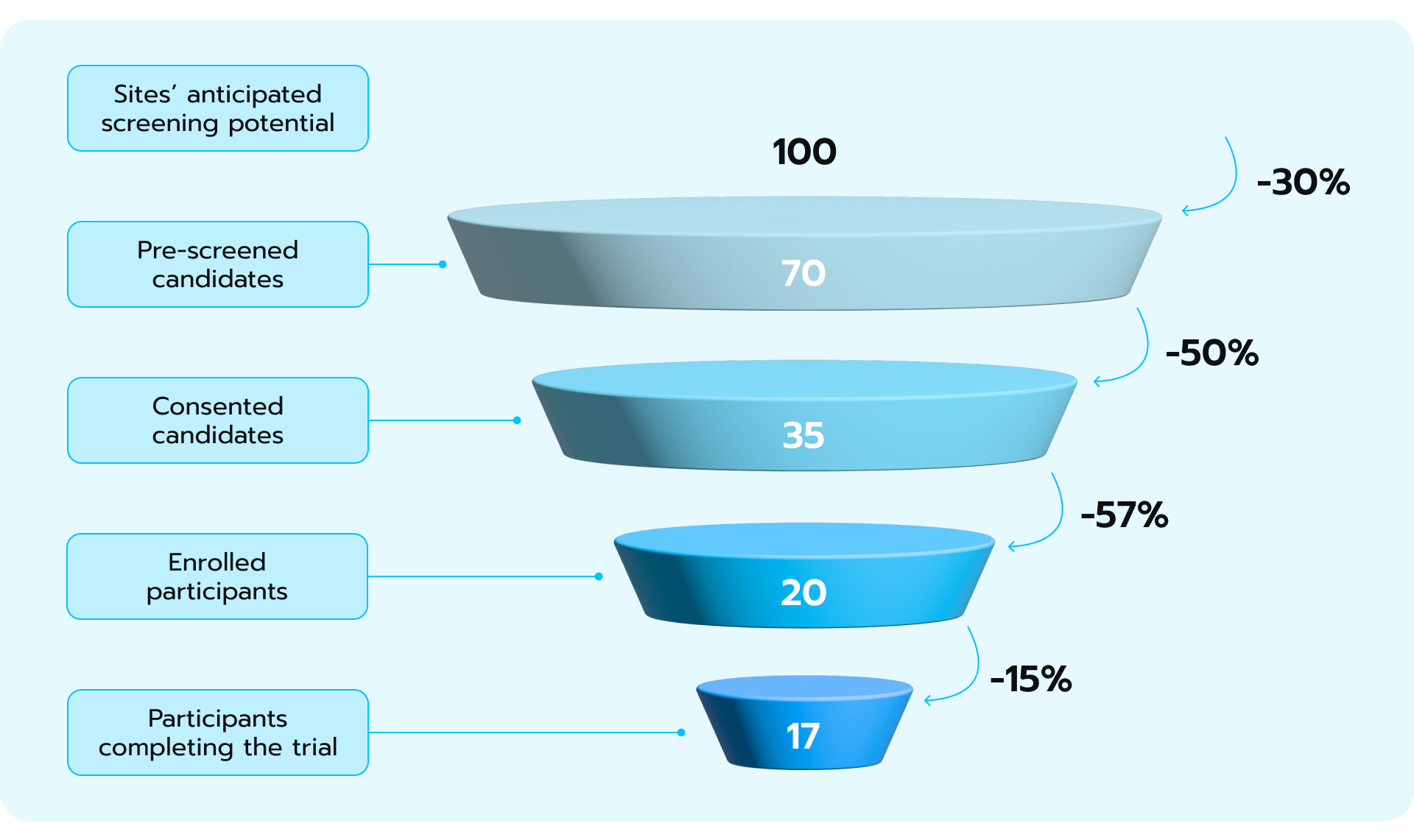 Figure 2. Patient recruitment funnel implies that the number of anticipated patients in a clinical trial will decrease over time.
Figure 2. Patient recruitment funnel implies that the number of anticipated patients in a clinical trial will decrease over time.
As Pascale Wermuth underlines in his research, even after randomization, high attrition rates threaten trial integrity. The dropout rate, which tends to climb with longer trials, can weaken the study’s ability to detect meaningful results. Markedly, the number of patients recruited for clinical trials is heavily influenced by the nature and severity of the disease. In 2021, a chief medical officer from a biotechnology company highlighted that fewer than 10% of cancer patients took part in clinical trials of any kind. The field of oncology stands out as an area where many trials are terminated prematurely due to inadequate patient enrollment. A comparable situation exists for patients suffering from central nervous system disorders or cardiovascular diseases (see Fig. 3).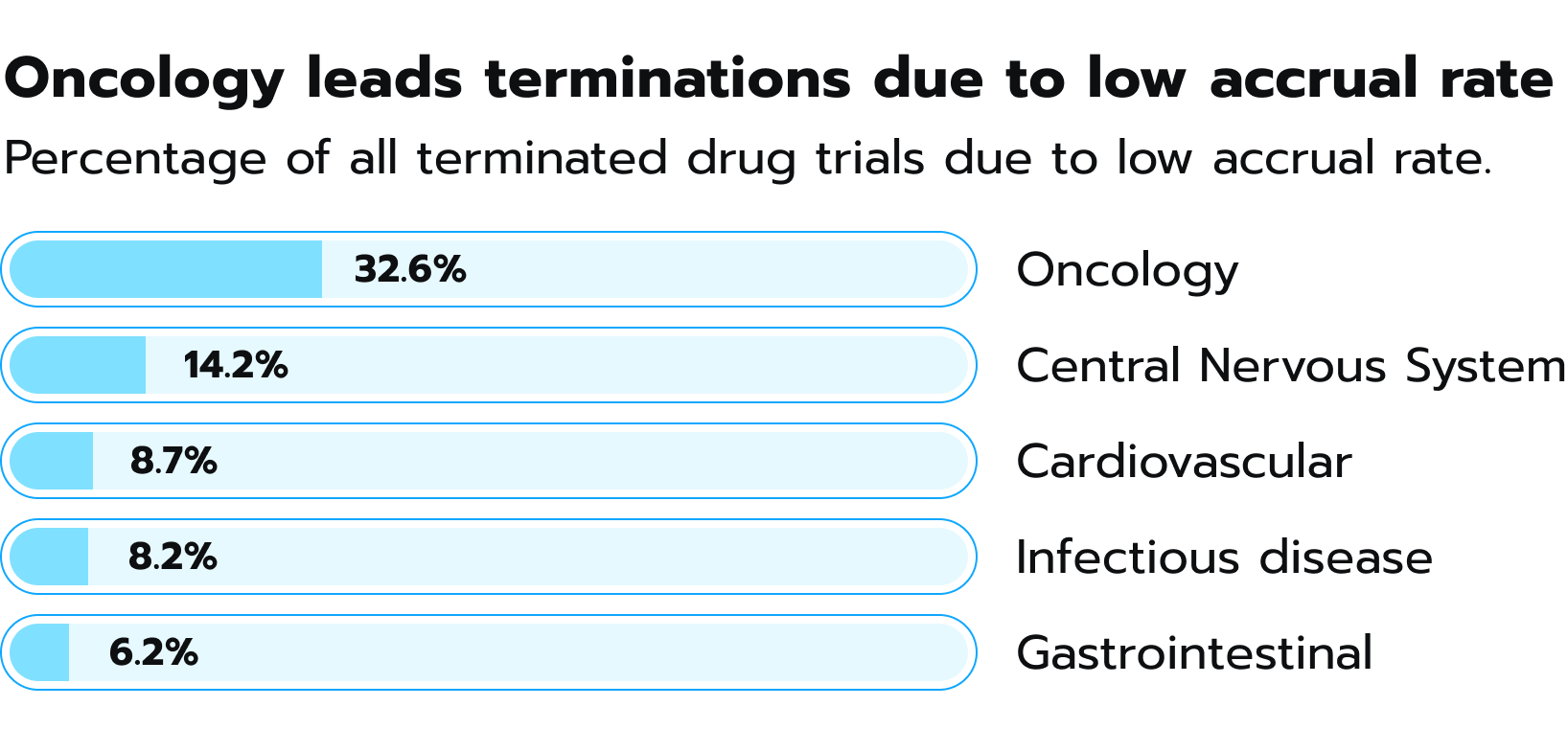 Figure 3. Percentage of terminated clinical trials because of low accrual rate, according to GlobalData.
Figure 3. Percentage of terminated clinical trials because of low accrual rate, according to GlobalData.
Interestingly enough, patient recruitment becomes more difficult as trials progress due to the increasingly specific requirements for participation. Phase I trials with healthy volunteers and broad criteria present the fewest hurdles. Phase II, which introduces the drug to actual patients for the first time, presents the most daunting challenge because of the stringent criteria needed to assure patient safety and accurate evaluation of the drug’s effects in this specific population. This moment presents a high risk of premature termination of the study since almost 53% of clinical trials end in phase II due to low patient accrual (see Fig. 4).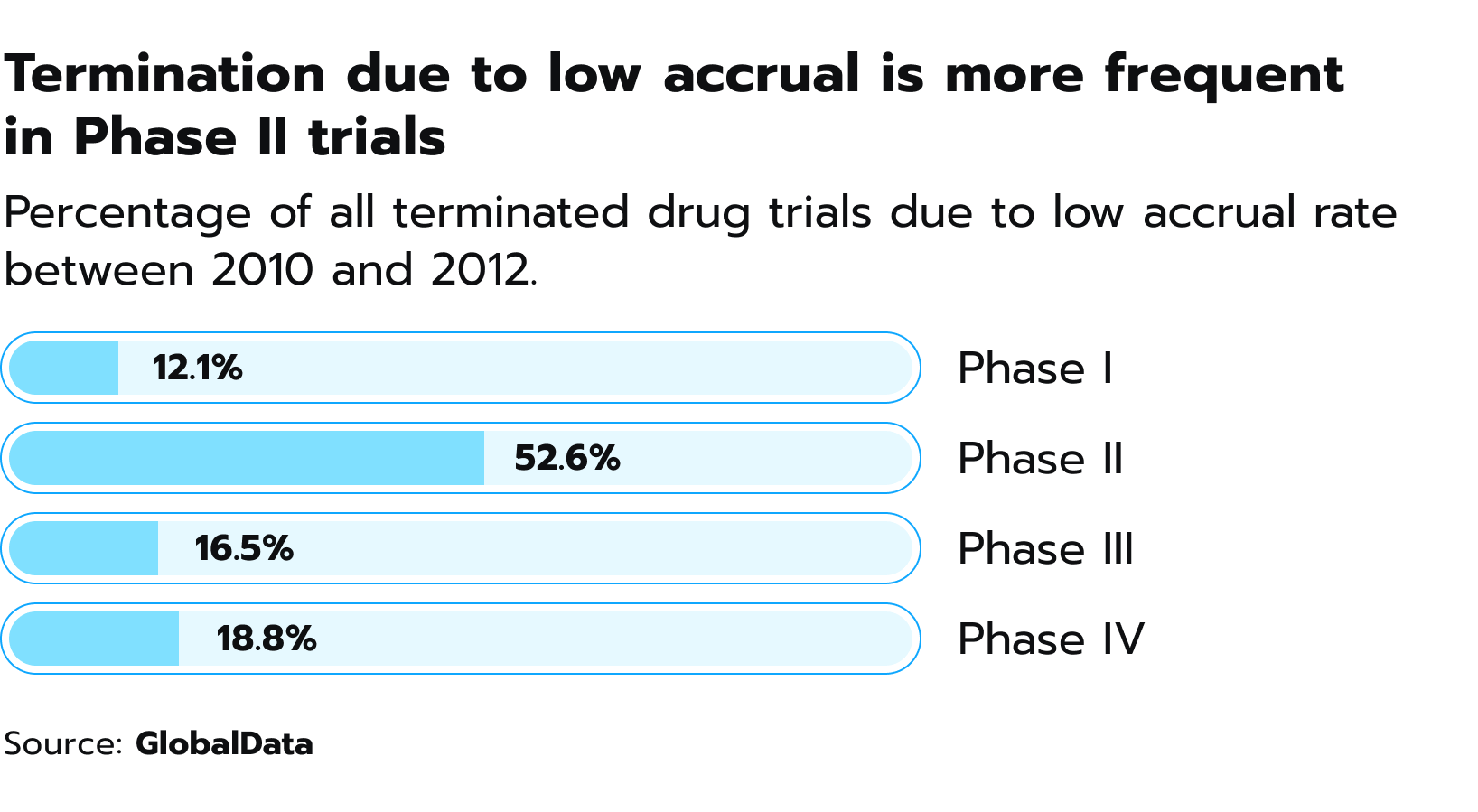 Figure 4. Percentage of drug trials that were terminated between 2010 and 2021 due to inadequate recruitment of patients.
Figure 4. Percentage of drug trials that were terminated between 2010 and 2021 due to inadequate recruitment of patients.
As trials move to Phases III and IV, the need for more representative patient populations necessitates broader inclusion criteria, which can ease recruitment to some extent. However, the sheer number of participants needed in later phases also stands as a challenge. Here is a comparative tables showing differences in recruitment limitations during each phase of clinical trials:
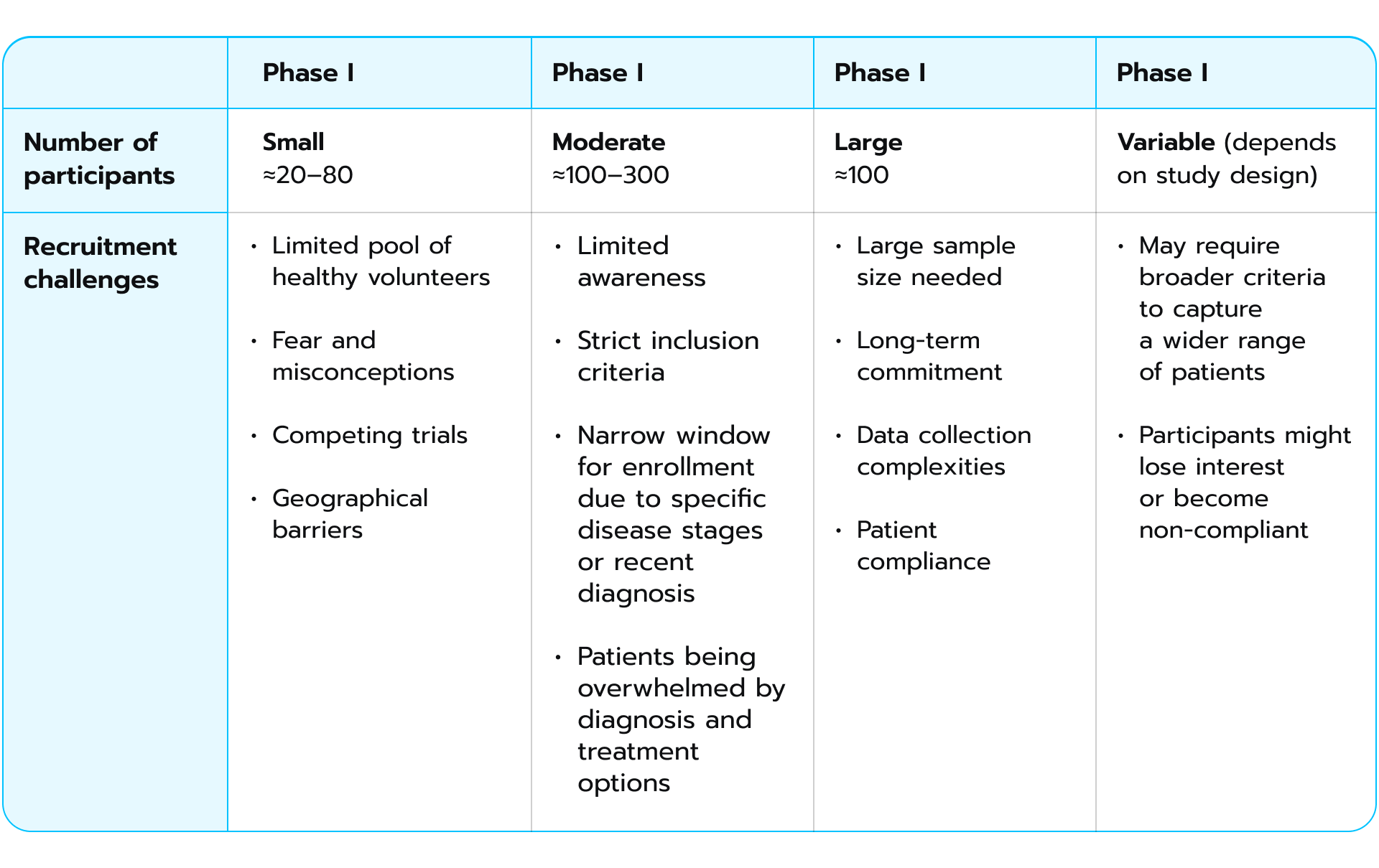 Recruitment strategies
Recruitment strategiesTo better grasp the rationale behind patient recruitment, let’s examine the strategies commonly employed for this purpose. There are two primary recruitment strategies frequently utilized in clinical trials. The first one leans on the existing pools of potential participants. These might imply working with patient registries that gather individuals with specific conditions, biobanks that store biological samples with corresponding data, or even clinical trial websites where individuals can self-enroll if their profile aligns with the study’s needs. This approach offers a head start, as there’s already a foundation of potentially interested individuals. Researchers usually opt for different ways to recruit participants. This number includes:
The second strategy for patient recruitment implies continuously searching for potential participants as they become eligible. Unlike its counterpart, which relies on existing pools, this method functions more like building a broader engagement net through various channels. This opportunistic recruitment strategy, while effective in reaching a larger pool of potential participants, presents its own set of challenges. Even when a condition like breast cancer affects a substantial number of people, identifying eligible individuals at the precise moment for trial participation can be surprisingly difficult. The window of opportunity is often quite narrow, particularly in critical settings like emergency care.
As an emerging force in healthcare and pharmaceuticals, generative AI can advance patient recruitment and accelerate the development of new therapies and interventions. According to McKinsey, the adoption of this technology could inject a substantial boost of $13-25 billion into clinical development within the pharmaceutical industry alone, with the potential to escalate to a total gain of $110 billion in the foreseeable future (see Fig. 5).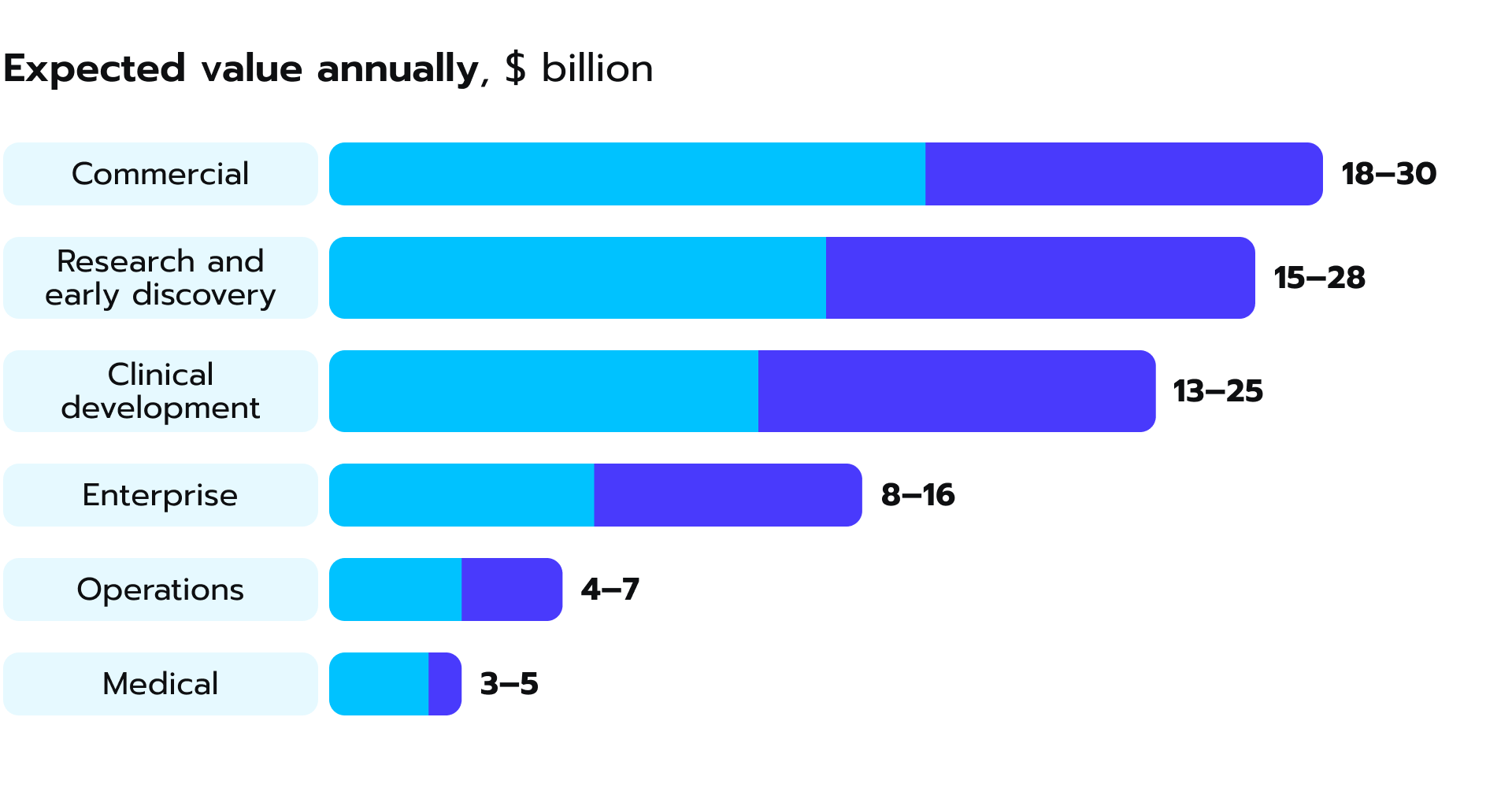 Figure 5. Generative AI holds great potential to bring value to clinical development, a McKinsey research suggests.
Figure 5. Generative AI holds great potential to bring value to clinical development, a McKinsey research suggests.
Generative AI’s promise to transform patient recruitment is rooted in its ability to address several key hindrances. Firstly, it can tackle the issue of bottlenecked efficiency. Traditional methods of sifting through medical records and identifying potential participants are time-consuming and resource-intensive. Generative AI, however, can analyze vast datasets of EHRs or other data points in a fraction of the time. Its algorithms can identify potential participants who meet specific trial criteria with great speed and accuracy.
Secondly, generative AI serves as a powerful tool for enabling an adaptive trial design, as it can continuously analyze patient data and adjust trial protocols based on real-world data (RWD). This dynamic approach allows researchers to optimize trial parameters, such as dosage levels, treatment durations, and inclusion criteria, based on evolving patient responses and emerging trends. Consequently, AI adoption accelerates the pace of clinical research and drives patient-centricity, a study recently published in Nature underlines. Below are several examples of how generative AI helps streamline the patient recruitment process.
Generative AI, equipped with Natural Language Processing (NLP) and Machine Learning capabilities, can automate the evaluation of eligibility criteria against a participant’s medical history data or other relevant data sources. Its integration eliminates the need for time-consuming manual review and frees up valuable time for researchers and clinical staff. Imagine a scenario where AI can pre-screen hundreds of potential participants within minutes. This expedites the entire recruitment process and minimizes the risk of errors.
Recently, following the advance of new similar solutions, researchers have developed a model called TrialGPT. It is designed to improve matching patients with suitable clinical trials. This system uses large language models (LLMs) to analyze patient medical records and compare them to the eligibility criteria for different trials. Initially, it was trained on data of 184 patients and 18,238 annotated clinical trials. Importantly, TrialGPT doesn’t just provide a yes or no answer, but also explains its reasoning in detail.
The researchers tested TrialGPT on a large dataset of patients and clinical trials, and found that it performed well. TrialGPT’s explanations closely matched those of human experts, and the system was effective at ranking trials and excluding those that patients wouldn’t be eligible for (see Fig. 6).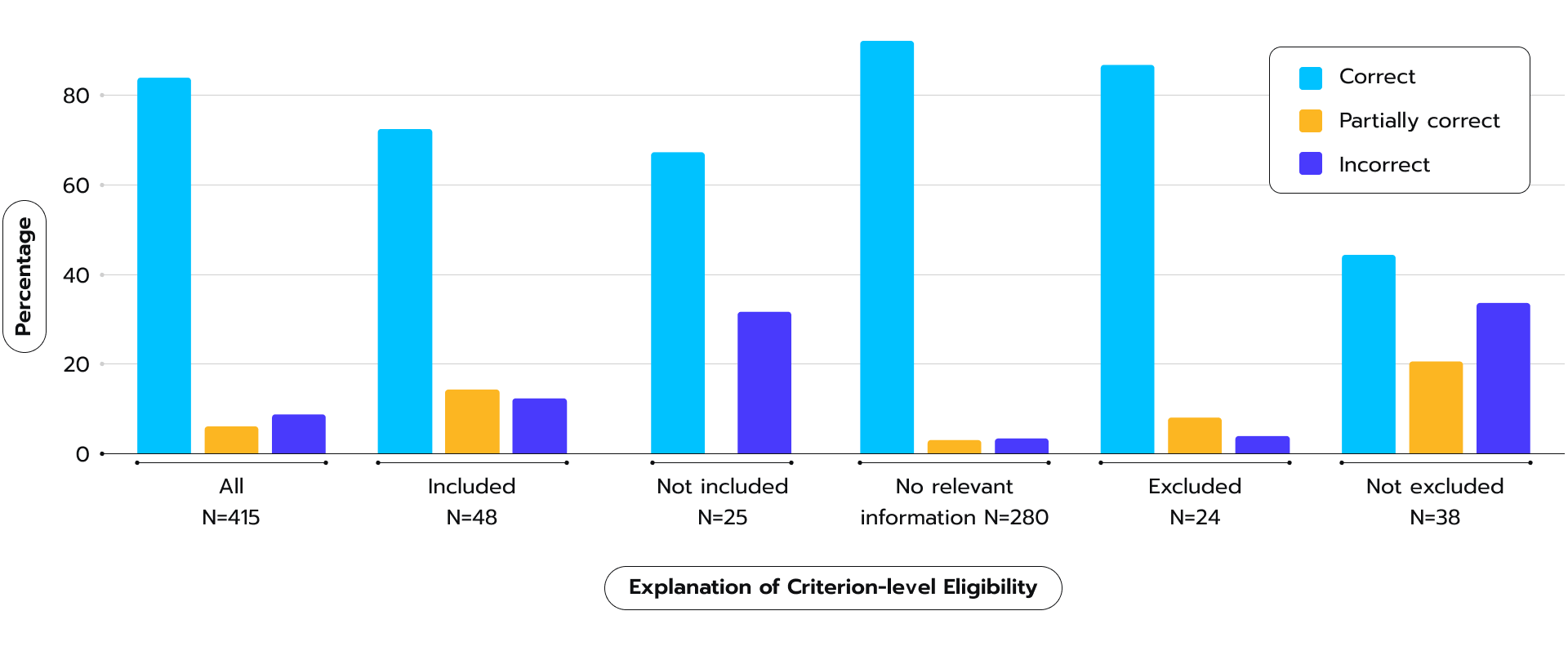 Figure 6. TrialGPT’s performance in generating relevance explanations, categorized as correct, partially correct, or incorrect, as per 2023 study.
Figure 6. TrialGPT’s performance in generating relevance explanations, categorized as correct, partially correct, or incorrect, as per 2023 study.
While there were still some errors due to limitations in the underlying language models, the researchers believe this technology has great potential. They suggest future research should focus on integrating TrialGPT into real-world clinical trial matching processes to streamline the system and improve efficiency. Yet, the gold standard of such tools evaluation should still be developed in the future.
Clinical trial sites are often overburdened with administrative tasks associated with participant screening. In that case, generative AI chatbots or virtual assistants can be deployed to handle the initial stages of this process. These AI-powered tools can communicate and ask questions based on the trial’s criteria, collect basic data from potential participants, and even schedule preliminary appointments. Overall, this approach can reduce the workload of a research team. The 24/7 availability of AI chatbots further drives accessibility for potential participants, particularly those with scheduling challenges or a preference for virtual interaction. myTrialsConnect, a new AI-enabled patient recruitment and engagement platform, serves as one of the numerous examples of how generative AI creates new ways of communication with potential participants in clinical trials.
Participant dropout during a clinical trial can greatly delay research and inflate costs. Machine Learning, with its analytical prowess on past clinical trial data, can determine patterns associated with early participant withdrawal. They might reveal factors like long commuting time to the trial site, complex medication schedules, lack of support systems, or even unforeseen side effects experienced by participants early on in the trial.
This predictive power allows researchers to develop targeted interventions to improve retention rates and guarantee that a sufficient number of participants complete the study. For example, AI might identify participants who live far from the trial site and suggest offering travel stipends or remote monitoring options. Besides, AI can help predict potential roadblocks or delays in the recruitment process itself, which enables researchers to proactively address these challenges and maintain the trial’s timeline. Imagine being able to anticipate a slowdown in participant enrollment and adjust your outreach strategy accordingly. This can be partly achieved by the deployment of AI.
For decades, clinical trial failure rates have remained high. They led to a slowdown in the development of new medications despite increasing investments by pharmaceutical companies in research and development (R&D). This problem is particularly acute for complex, innovative treatments targeted towards smaller and more diverse patient populations. Traditional clinical trials simply did not have the analytical power, adaptability, or efficiency needed to effectively evaluate new treatment options, a research underscores (see Fig. 7). Besides, a lack of unified efforts exacerbated the problem. For instance, the U.S. Food and Drug Administration (FDA) set up a demand for obligatory reporting of demographic subgroups in clinical trials less than 10 years ago.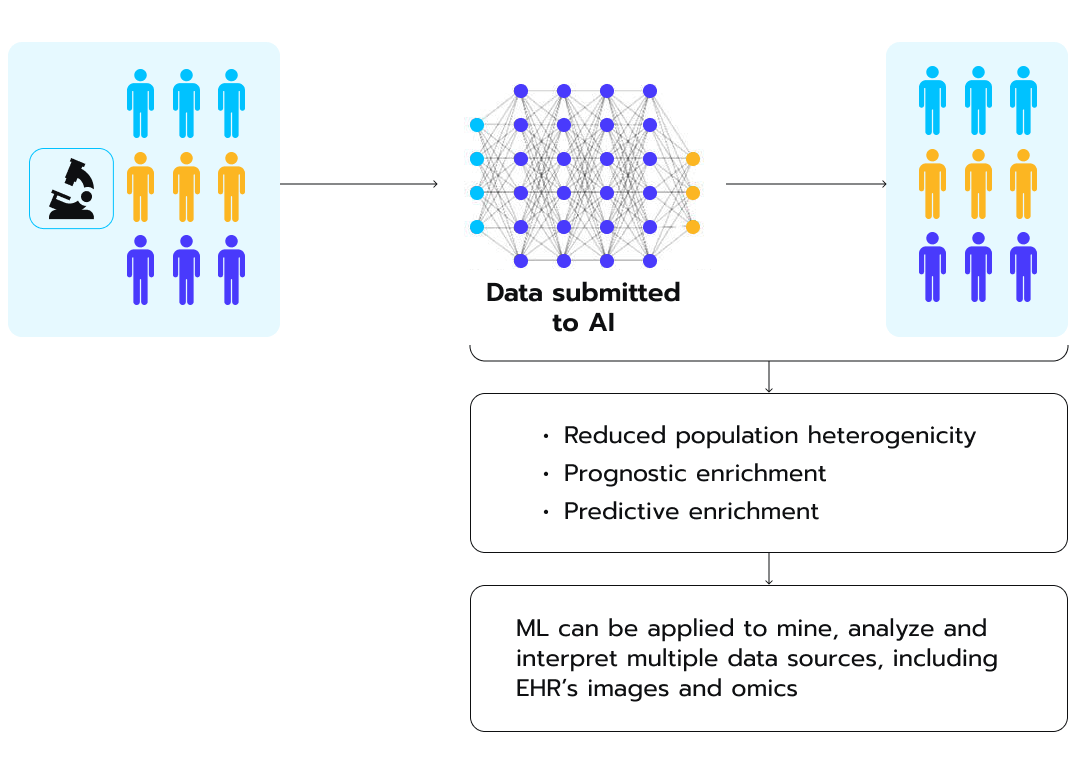 Figure 7. How AI can drive greater diversity during patient recruitment, according to a recent study.
Figure 7. How AI can drive greater diversity during patient recruitment, according to a recent study.
The process starts with AI models ingesting diverse data submitted from heterogeneous populations, as depicted in the image. Through automated analysis and integration of disparate EHR, genomic, medical imaging, and other datasets, AI extracts nuanced patterns and generates actionable insights. These predictive intelligence capabilities can then be applied to reduce population heterogeneity and strategically enrich study cohorts.
Even after identifying viable candidates, one of the biggest hurdles in recruitment is actually reaching and engaging those potential participants. Conventional outreach methods like mass media advertising, mailed brochures, or call centers are often seen as inefficient “spray and pray” approaches.
AI opens up new channels for intelligent, targeted trial awareness and outreach. Basing its conclusions on vast data sources, it can pinpoint the ideal messaging, content format, communication platform, and timing to resonate with specific patient segments.
Consider a scenario where an AI model analyzes not just a potential participant’s medical history, but also their online browsing and app usage patterns. This analysis could reveal that the individual is more likely to respond to visual content and prefers social media engagement. Armed with this knowledge, the AI could automatically generate a concise explainer video specifically for the participant. The video, hosted on a relevant social media platform, would highlight the key benefits of the clinical trial in a relatable narrative style. This personalized approach goes beyond simply conveying information – it tailors the message to resonate with the individual’s preferred communication style.
Contrast that level of personalized, contextual nurturing with a generic study flier lost in a stack of medical bills. The increased engagement rates facilitated by AI’s intelligent multichannel awareness strategies can dramatically boost recruitment funnel conversion.
While generative AI offers a powerful tool for automating eligibility evaluation and streamlining screening, it is essential to emphasize the importance of human oversight. No matter how sophisticated, AI models should not replace the expertise of scientists and medical professionals. Investigators, with their in-depth knowledge and experience, should review all AI-generated recommendations and make the final decisions regarding participant eligibility or other crucial aspects of the recruitment process. Here are three essential examples of challenges to wide-scale generative AI adoption in patient recruitment:
AI models are only as good as the data they are trained on. Inconsistent data formats, missing information, or errors within EHRs can lead to inaccurate predictions and biased outcomes. Imagine an AI model trained on data where certain ethnicities are underrepresented – its ability to select suitable participants from those demographics would be severely hampered. Standardizing data collection and safeguarding its quality across different healthcare institutions is a critical but complex task. Collaboration between healthcare providers, technology vendors, and regulatory bodies is essential to develop and implement common data standards. Additionally, data cleaning and normalization techniques can be applied to improve the quality of existing EHRs data.
Strict data privacy regulations like Health Insurance Portability and Accountability Act (HIPAA) and General Data Protection Regulation (GDPR) govern the collection, storage, and use of patient data. Integration of AI models into clinical trials requires careful consideration of these regulations as it is imperative for the protection of participant privacy throughout the recruitment process. Researchers need to implement robust data security measures and anonymization techniques to safeguard patient information. Also, obtaining informed consent from participants regarding how their data will be used for AI-powered recruitment is essential for building trust.
Transparency on how AI uses patient data helps build responsible communication with participants and the broader medical community. In response, researchers can offer high-level explanations of how AI is used in the recruitment process, without divulging confidential details about the specific algorithms. Additionally, independent audits and reviews of AI models can uphold responsible data practices.
The evolving nature of AI creates a need for the development of robust governance frameworks to guide its use in clinical trials. While the regulatory landscape for AI remains largely uncharted in the US, the European Union (EU) made a significant stride in this sphere when it adopted the AI Act on March 13, 2024. This pioneering legislation represents the first comprehensive effort to establish a legal framework governing the development, deployment, and use of AI systems across various sectors, including healthcare and pharmaceuticals.
A future where AI helps rethink patient recruitment and unlocks deeper insights from medical data is no longer science fiction. From study planning to patient enrollment and retention, AI offers multiple ways to transform clinical trials. Collaborative efforts between researchers, pharmaceutical companies, and regulatory bodies drive positive dynamics in this domain. The time is ripe for the exploration of new possibilities.
Lay the groundwork for your AI excellence with Avenga. Form a strong collaboration to future-proof your organization and promote enduring resilience: contact us.
Ready to innovate your business?
We are! Let’s kick-off our journey to success!