How computer vision reinvents clinical trials today.
Over the last few years, Artificial Intelligence (AI)-enabled clinical trials have proven to be effective in lowering the financial barrier to new therapies’ approval. As one of the organizations supporting the trend, National Robotics Engineering Center at Carnegie Mellon University in Pennsylvania, in collaboration with Columbia University, created a computer vision algorithm to monitor patients in clinical trials. The system provided an opportunity to observe children diagnosed with Spinal Muscular Atrophy (SMA) without the need for hospital admissions or clinician visits.
The advance in computer vision pushes the line further towards better patient selection, recruitment, and retention during clinical trials. In this article, we will explore how AI, particularly computer vision, can challenge clinical development in the near future.
Why are modern clinical trials so archaic?
It has been 80 years since the first double-blind controlled trial was conducted in Great Britain. More than 1,000 patients arrived from different corners of the country to help researchers investigate patulin treatment for the common cold. According to the statistics, British office and factory workers traveled at least two hours by train or car to reach the location where the clinical trial took place. Clinical investigators described all observations and manually documented valuable bits of information. It took one and a half years to synthesize the results and create a report on the gathered data.
Although almost a century has passed since the first double-blind controlled trial, clinical development showed relatively slow progress in improving efficacy and safety standards. Even today, patients are likely to travel long distances to get to the site and have limited access to the trial results. Likewise, companies struggle to get accurate results with insufficient diagnostic tools and lag behind because of paper-based recording.

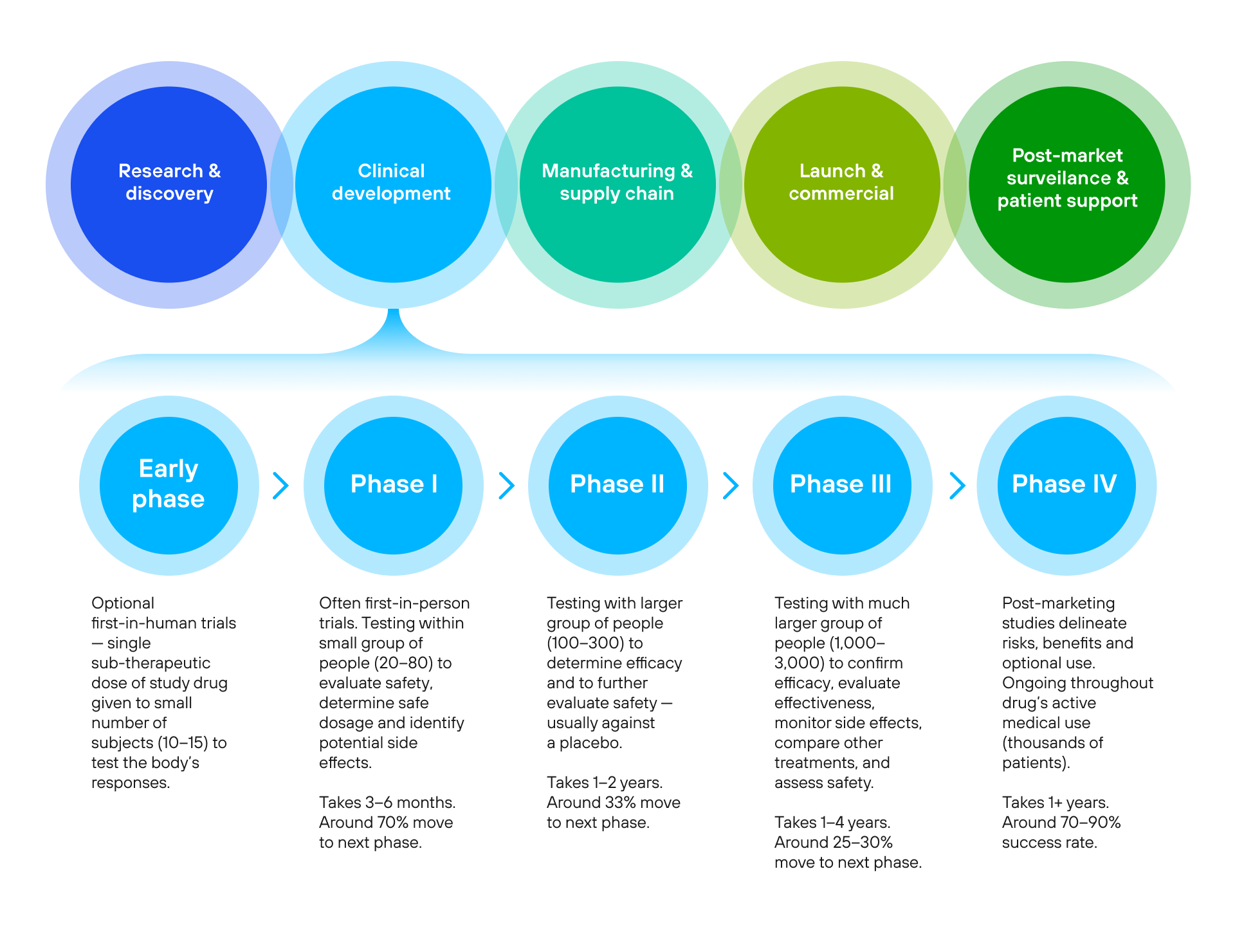 Figure 1.
Figure 1. 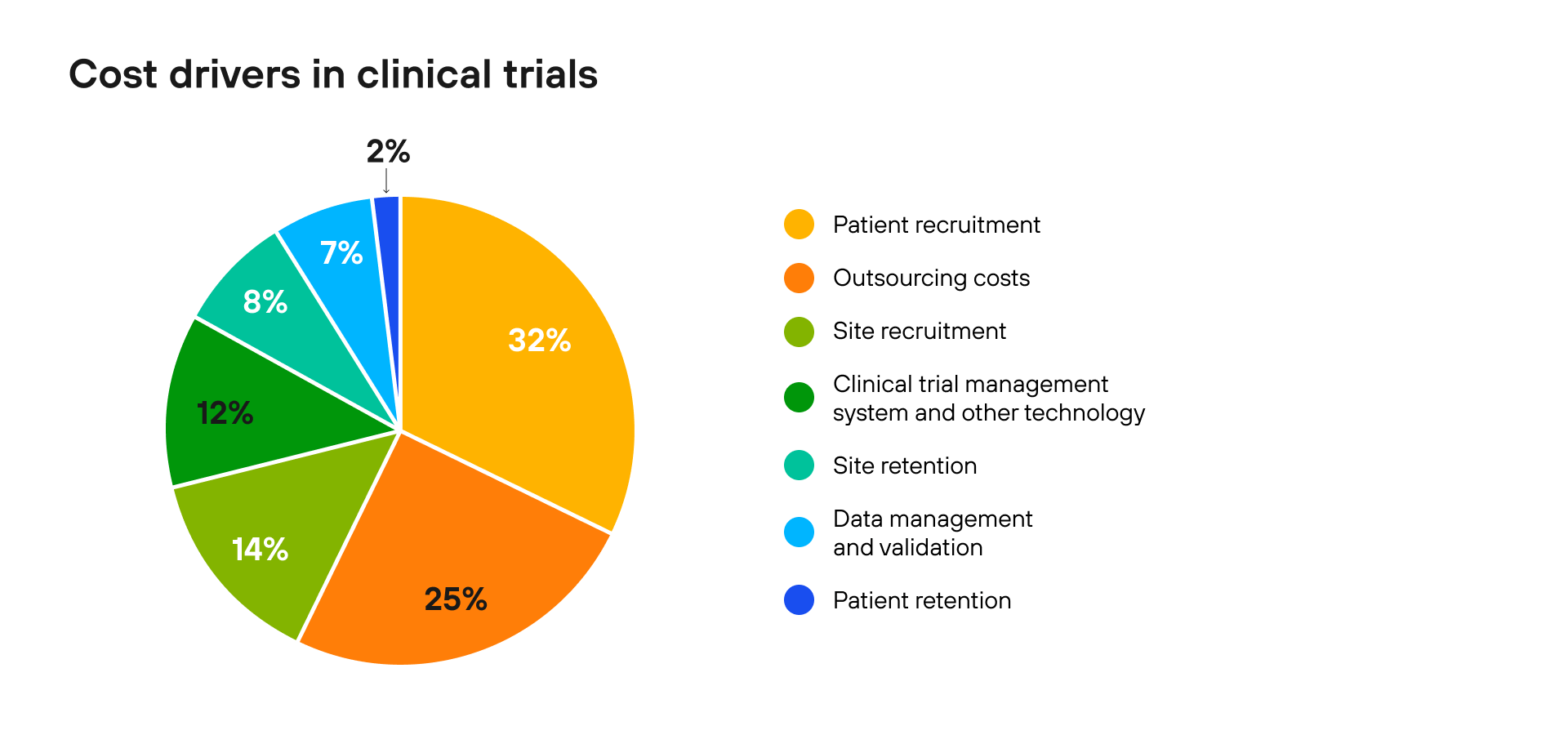 Figure 2.
Figure 2. 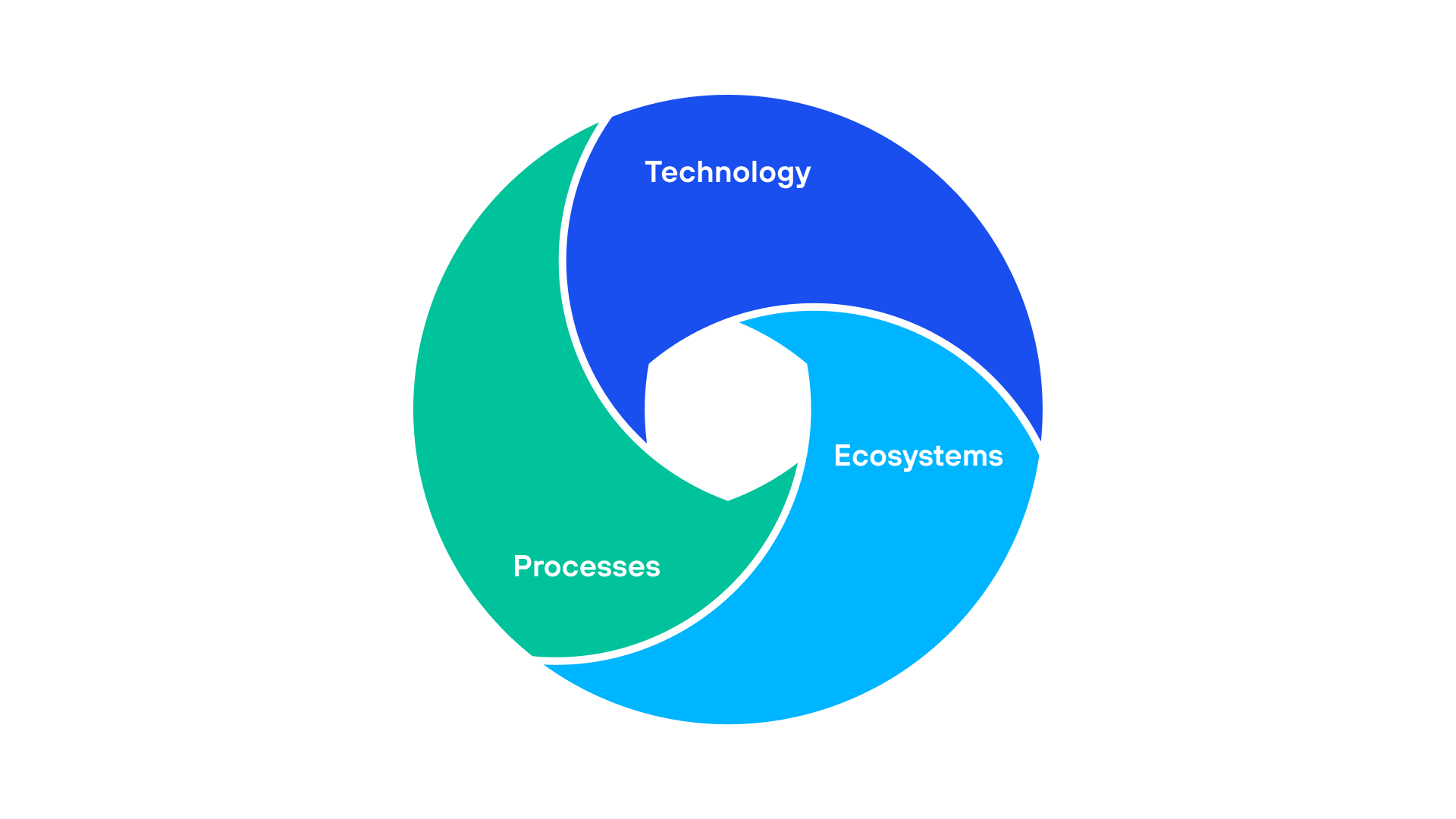 Figure 3. Enhance patient journey by prioritizing processes, technology, and ecosystems, as highlighted in a
Figure 3. Enhance patient journey by prioritizing processes, technology, and ecosystems, as highlighted in a 






